HCOOH + CH₃CH₂OH + H₂O: Simple Reaction, Big Impact in 2025
Have you ever mixed things together just to see what happens? In chemistry, people do that all the time. One very interesting mix is when formic acid (HCOOH) and ethanol (CH₃CH₂OH) come together in water (H₂O). These three look simple, but when they meet, something cool happens. They make a new chemical called ethyl formate.
In this post, we’ll explain what goes on when these three mix. We’ll keep it simple, fun, and easy to understand—even if you’re new to chemistry. Let’s explore the reaction and learn why it matters.
Meet the Molecules: HCOOH, CH₃CH₂OH, and H₂O
Let’s look at the three things we’re mixing.
HCOOH – Formic Acid
This is a very small acid. It’s in the sting of ants! It’s sharp and strong, even though it looks simple. People use it in farming and in labs.
CH₃CH₂OH – Ethanol
You may know ethanol as the kind of alcohol in drinks. But it’s also used as a cleaner and fuel. It’s made from plants and is safe in small amounts.
H₂O – Water
We all know water. It’s in rivers, lakes, and our bodies. It helps many reactions happen. In this case, water also forms during the process.
The Big Reaction: HCOOH + CH₃CH₂OH + H₂O

Now let’s see what happens when you mix them.
What Do You Get?
When formic acid and ethanol mix, they can make ethyl formate, which smells like fruit. Water is also made.
The Reaction:
HCOOH + CH₃CH₂OH → HCOOCH₂CH₃ + H₂O
- HCOOH = formic acid
- CH₃CH₂OH = ethanol
- HCOOCH₂CH₃ = ethyl formate (an ester)
- H₂O = water
This reaction is called esterification. That means an acid and an alcohol come together and make something called an ester, along with water.
Why Does This Reaction Happen?
Let’s break it down step by step.
What Is an Ester?
An ester is a new compound made when an acid and alcohol mix. Esters smell sweet. You can find them in fruit, gum, and even some perfumes.
What Helps the Reaction?
Heat or an acid (like sulfuric acid) helps this reaction go faster. Without help, the mix might take a long time to change.
What Does Water Do?
Water is made during the reaction, but it can also stop the reaction if there’s too much. In labs, people often remove water to keep the reaction moving forward.
Where Do We Use Ethyl Formate?

A Chemical with Many Uses
Even though this seems like a small reaction, the product—ethyl formate—is very useful.
In the Factory
- It’s used to clean things like plastics and rubber.
- It can be used to kill bugs in grain stores.
- It helps make other chemicals too.
In Perfume and Food
- Ethyl formate smells like rum or fruit.
- It’s used in perfumes and body sprays.
- It’s used to give candy and drinks a nice smell or taste.
In Fuel Research
Scientists study it to see if it can be used in fuels. It burns clean and may help make greener fuel one day.
Innovation News DualMedia: The Future of Digital News
Problems That Can Happen
Like many things, this reaction doesn’t always go smoothly.
Water Gets in the Way
Since water is made in the process, it can build up and stop more ester from forming. Chemists use tricks to remove water as it’s made.
Needs a Little Help
The reaction is slow without a helper. A strong acid like sulfuric acid speeds things up. But acids can be dangerous, so people must be careful.
Clean Ingredients Matter
If the formic acid or ethanol isn’t pure, the result might not be clean. You could end up with other stuff you didn’t want.
Is This Reaction Safe?
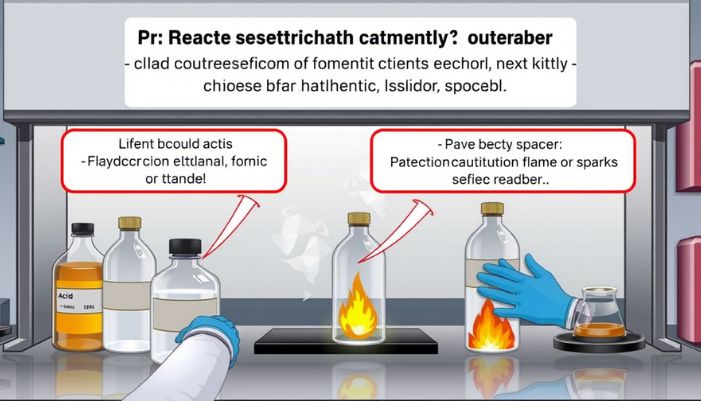
This reaction is safe in small amounts if done the right way. But let’s talk about what to watch out for.
Be Careful With Formic Acid
Formic acid can hurt your skin or eyes. It also smells bad. Wear gloves and goggles when working with it.
Watch Out for Ethanol
Ethanol burns easily. Don’t use it near flames or sparks. It also dries out your skin.
Use Good Airflow
Do this reaction in a place with good air. A fume hood is best. This keeps strong smells and fumes away from your face.
What This Reaction Teaches Us
Even simple reactions can be powerful. This one shows how we can turn small things into something new and useful.
- Formic acid + ethanol = ester + water
- This is called an esterification reaction
- The product, ethyl formate, is used in real-world jobs
- Heat or acid helps the reaction move faster
- Water can slow it down, so people remove it as they go
Frequently Asked Questions (FAQs)
Final Thoughts
The reaction between HCOOH, CH₃CH₂OH, and H₂O is a great example of how tiny molecules can make something new and useful. With the help of a little heat or acid, you get ethyl formate—a sweet-smelling liquid found in everyday stuff like food and perfumes.
This is the kind of reaction that makes chemistry exciting. It shows how mixing the right things, under the right conditions, can lead to something that smells good, works well, and helps in many areas of life.
Even if you don’t work in a lab, it’s pretty amazing to know that the stuff ants make (formic acid) and the alcohol in drinks (ethanol) can join forces to make something so useful. Cool, right?